


























Tap Tap to Zoom
Product Discontinued by Manufacturer
Howard Leight Dual Layer Face Cover has been discontinued by Howard Leight and is no longer available. Our product experts have helped us select these available replacements below.You can also explore other items in the Lab Safety & Apparel, Tools, Survival Gear, Personal Protective Equipment, Safety Masks & Respirators yourself to try and find the perfect replacement for you!
Product Info for Howard Leight Dual Layer Face Cover
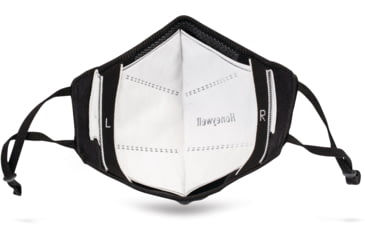
This face mask product is designed to withstand multiple washes, and when used with a disposable insert provides the wearer with an extra layer of mouth and nose coverage beyond a common cloth face covering. The filter insert has been shown by a third-party testing facility to block up to 97% of 3-micron-sized non-viable particles and 0.1-micron-sized aerosolized particles. WARNING: The product has not been FDA (Food and Drug Administration) cleared or approved. The face mask has been authorized by FDA under an Emergency Use Authorization for use as source control by the general public as well as by healthcare personnel in healthcare settings as to help prevent the spread of infection or illness during the COVID-19 pandemic. Source control refers to the use of a face mask over the mouth and nose to contain that individuals respiratory secretions to help prevent transmission from infected individuals who may or may not have symptoms of COVID-19. There has not been testing of the effectiveness of the disposable insert when placed in the Face Mask. Accordingly, the Face Mask is not a respiratory protective device, nor a substitute for filtering face piece respirators or for surgical face masks. This product is not for medical use or for use in a high infection risk clinical setting. It is not a surgical mask and is not intended to be used to provide liquid barrier protection or for high-risk aerosol generating procedures. The face mask is authorized only for the duration of the declaration that circumstances exist justifying the authorization of the emergency use of medical devices, including alternative products used as medical devices, during the COVID-19 outbreak, under section 564(b)(1) of the Federal, Food, Drug, and Cosmetic Act, 21 U.S.C. 360bbb-3(b)(1) unless the authorization is terminated or revoked sooner. This product has not been tested against COVID-19.![]()
Features of Dual Layer Face Cover:
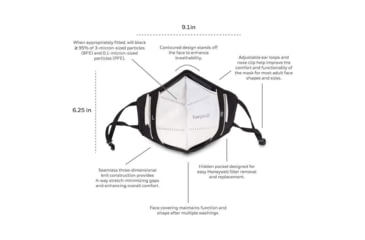
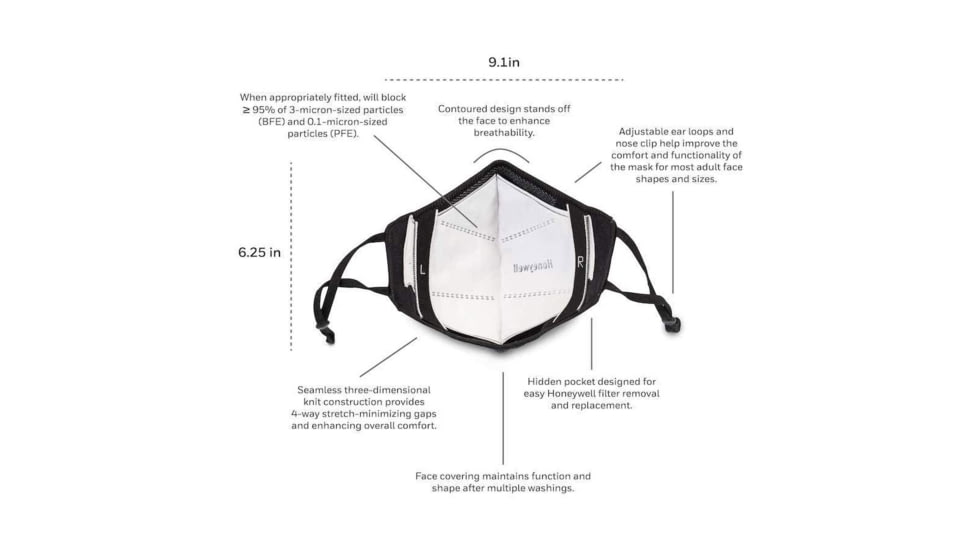
- COMFORTABLE: Seamless three-dimensional knit construction provides 4-way stretch-minimizing gaps and enhancing overall comfort.
- ADJUSTABLE: Ear loops and nose clip are adjustable.
- REPLACEABLE PROTECTIVE INSERTS: Hidden pocket designed for easy Howard Leight insert removal and replacement.
- ENHANCE BREATHABILITY: Contoured design stands off the face to enhance breathability.
- REUSABLE: Face covering maintains function and shape after multiple washings. Remove insert prior to washing.
- Face Cover Materials: Nylon, Polyester, Spandex;
- Straps Materials: Polyester, Spandex; Insert: Polypropylene, Polyester
Package Contents:
- Howard Leight Dual Layer Face Cover
Related Products to Howard Leight Dual Layer Face Cover
Howard Leight Dual Layer Face Cover Unavailable & Discontinued Models
List of Unorderable Models
- Howard Leight Dual Layer Face Cover with 8 Replaceable Filters, Light Gray, RWS-50107 , MPN: RWS-50107 , UPC: 883940394183 , Code: HL-PRP-HON001-RWS-50107
- Howard Leight Dual Layer Face Cover with 8 Replaceable Filters, Dark Gray, RWS-50111 , MPN: RWS-50111 , UPC: 883940398365 , Code: HL-PRP-HON001-RWS-50111
- Howard Leight Dual Layer Face Cover 4-Pack with 32 Replaceable Filters, Dark Gray, RWS-50112 , MPN: RWS-50112 , UPC: 883940398426 , Code: HL-PRP-HON001-RWS-50112
























